PTM&W General FAQs
- How Critical Are Mix Ratios?
- What Is The Best Method For Measuring Mix Ratios?
- Can Pot Life Be Adjusted By Changing The Mix Ratio?
- What Factors Effect Pot Life And Cure Time?
- What Is An Exothermic Reaction?
- What Is The Difference In The Terms: Pot Life, Gel Time, Working Time, Tack-Off Time, Etc.?
- Do Epoxies And Urethanes Outgas?
- Why Do High-Temperature Epoxies Change Color After Exposure To Elevated Temperatures?
- What Is The Difference Between Glass Transition Temperature And Heat Deflection Temperature?
- Can A Tool Be Used Above Its Glass Transition Or Heat Deflection Temperature?
- How Important Is Post-Curing For High-Temperature Tooling?
- Can Epoxies Be Bonded To Cured Epoxy?
- How Can Empty Containers Be Disposed?
How Critical Are Mix Ratios?
Catalytic-cure polymers, like polyesters, will cure with any amount of catalyst added. A catalyst just starts the reaction. The more catalyst, the shorter the pot life and faster the cure time. Epoxies and urethanes, with a few exceptions, are designed to cure at an exact mix ratio, which allows each molecule of resin will crosslink with a molecule of hardener. Off-ratio mixes result in excess resin or hardener molecules that have nowhere to attach, which results in deteriorated properties.
Each resin/hardener mixture has a tolerance for error. Generally speaking, for epoxies it is safe to use ratios within 10% of the exact ratio and within 5% for urethanes. Beyond these tolerances, the systems may still cure, but with degraded properties. This is especially true for high-temperature systems where the first property affected from being off ratio is the Heat Distortion Point (HDT). If the system is far enough off ratio, it will only partially cure and be obviously unusable.
It is important to note that the target mix ratio should be the one that is published. The tolerance range allows for slight errors in measurement. To intentionally measure off ratio is never advised. Some epoxy hardeners are formulated to be “variable-ratio hardeners”. The published ratios for these hardeners will show a range of acceptable mix ratios.
Be aware of smaller batch sizes where more accuracy is required to be on ratio. Likewise, low-ratio systems like 100:5 or 100:10 need to be weighed more accurately then high-ratio systems like 100:50.
What Is The Best Method For Measuring Mix Ratios?
Mix ratios are stated in two ways, by weight and by volume. Weighing is the most accurate method of measuring ratios. Weight ratios are designated as parts-by-weight (pbw) or parts-per-hundred (pph). The weight ratio will be shown as 100:15 pbw, for example. This means every hundred parts of resin needs 15 parts of hardener for complete reaction. Example: A 300-gram batch of resin at 100:15 pbw mix ratio needs 45-grams of hardener.
Volumetric ratios are primarily used for dispensing equipment. These ratios are expressed in two ways: parts-by-volume (pbv) or by a ratio. A volumetric ratio could be stated as 100:25 pbv or 4:1 by volume.
Some, who do not have a scale for weight measurement, use graduated containers to measure by volume. This works where the ratios are even, like 1:1, 2:1, 4:1, etc. Volumetric measuring becomes a problem when the ratios are uneven, such as 100:17.35 pbv, or 5.75:1 by volume. Chances of being off ratio are increased by measuring volumetric ratios by hand.
Can Pot Life Be Adjusted By Changing The Mix Ratio?
Epoxies and urethanes are designed with one exact mix ratio. It is not recommended to lower the mix ratio during hot weather to increase the pot life. Likewise, ratios should not be increased during cold weather to speed up resin systems.
During hot weather it is best to mix smaller batch-sizes at the correct ratio, or use a slower-reacting hardener at its correct ratio. During cold weather, larger batches can be mixed, or a faster-setting hardener used to speed reaction time. Changing mix ratios to adjust pot life is always dangerous.
What Factors Effect Pot Life And Cure Time?
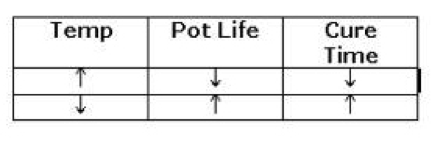
Factors that directly influence pot life and cure times are: Working temperature, mass of the mixed material, and the speed of the hardener. Pot life is measured in the lab at an ambient temperature of 25° C. (77° F.). There is a general rule-of-thumb for epoxies that for every 10° C. the temperature goes up, the pot life and cure time will be reduced by half. If it increases by 20° C., the times will be cut in half, and cut in half again. This rule is also generally true in reverse; for each 10° C. decrease in temperature, the pot life and cure time will double.
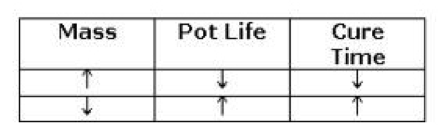
Pot life is measured in the lab for a certain mass of mixed material, usually 100-grams unless otherwise stated. As the mass of the mixed material increases, the pot life and cure time decrease. As the mass decreases, the pot life and cure time increase.
The choice of hardener affects the pot life and cure time of a system. One epoxy resin can be used with a large number of different speed hardeners to get a wide range of pot life and cure times. Please call us if a special resin/hardener combination is required for specific pot life and cure time requirements.
What Is An Exothermic Reaction?
Epoxies and urethanes (to a lesser extent) create heat when the resin and hardener crosslink, or chemically combine. This internally generated heat is called exotherm, and is a necessary by-product of the curing cycle. When exotherm becomes excessive it results in the resin boiling, smoking, shrinking and emitting strong odors.
Choosing correct hardener speed, controlling ambient temperatures, and limiting the mass of the mixed material are ways of minimizing exothermic reactions. If large mixes are required for a job, use a slower reacting hardener. If a specific hardener has to be used, mix smaller batch sizes. A mass of material will exotherm more quickly when left in a concentrated volume, than if it was spread over a larger area. When mixing large amounts of material, pot life can be extended by pouring off into smaller containers and working out of those.
If a mixing cup starts to exotherm, place the container either outside or in a well-ventilated area. Submerging in water will stop the exothermic reaction. Never place a container that is exotherming into a trashcan that contains combustible materials.
What Is The Difference In The Terms: Pot Life, Gel Time, Working Time, Tack-Off Time, Etc.?
Pot Life and Gel Time are used interchangeably – This measurement is made in the lab under controlled conditions, usually at 77° F. and in a 100-gram mass. This is usually tested in an XXX Gel Timer instrument, which rotates a wire hook in the liquid mixture until it thickens to the point where it reaches a predetermined torque level and shuts off. The machine then records the elapsed time.
Working Time – The time it takes for a mixture to reach a viscosity where it becomes unworkable. For example: the point where a laminating resin becomes too thick to wet-out fabric. The material may have not gelled at this point; it is just too thick to do the job for which it was intended.
Tack Time – The time for a mixture to cure to the point where it is “rubbery”. At this point you can leave a fingerprint on the surface of the system and it will not transfer to your finger. This is the ideal time to bond one layer of liquid epoxy or urethane to a cured layer, i.e., to laminate against a surface coat.
Tack-Off Time – The time for a system to become hard to the touch, with no tackiness. This is a good time to sand the surface, without gumming up the sandpaper. If an epoxy surface has reached this level, it must be sanded before another layer of liquid epoxy is bonded to it. At this point, the resin system is only partially cured. Demold Time – The time for a resin system to cure to the point where it can be removed from the mold without distortion. It is not fully cured at this time.
Cure Time – The time it takes a system to reach full properties. This time can be measured after a room-temperature or an elevated-temperature cure.
Do Epoxies And Urethanes Outgas?
The epoxies and urethanes we formulate do not contain solvents. Our systems are “100% solids”, which means all the ingredients, when combined at the proper mix ratio, react completely with no outgassing. Once cured, the chemicals are linked together and not able to come off as a vapor.
Why Do High-Temperature Epoxies Change Color After Exposure To Elevated Temperatures?
Most high-temperature epoxy systems will darken due to amine-based hardeners, which darken with heat. Do not confuse this darkening with a deterioration of properties. It is possible to char an epoxy laminate or casting if it is heated far above its maximum temperature. Some hardeners cause epoxies to have a dark-reddish cast after heat exposure. This is a normal reaction of certain hardeners. The epoxy has not been burnt.
What Is The Difference Between Glass Transition Temperature And Heat Deflection Temperature?
Both processes measure the same thing, the point where the cured resin goes through a change in its molecular structure. At this point mechanical properties decrease at an increasing rate and the coefficient of thermal expansion increases.
Heat Deflection Temperature (HDT) is a mechanical method of measuring this point. A cast bar measuring ½” X ½” X 5” is suspended between two points 4” apart. A load is applied halfway between the two suspension points. The entire apparatus is submerged in an oil bath, with the temperature of the oil raised at a set rate. When the cast bar deflects 0.010-inches under the load, the oil temperature is recorded as the HDT. This number is commonly reported at two load levels, 64 psi and 264 psi. Measuring HDT is a time consuming procedure that can tie up a lab technician for hours.
Note: Some companies report high HDT numbers by testing laminates instead of cast bars. Fiber reinforcement keeps the bar from deflecting even after the resin system has passed its HDT. These numbers do not accurately represent the capacity of a resin system. Glass Transition Temperature (Tg) is a computerized method of measuring the molecular change. There are three types of equipment used to determine Tg, by different measuring methods: Differential Scanning Calorimeter (DSC), Dynamic Mechanical Analyzer (DMA) and Thermal Mechanical Analyzer (TMA). The chemist decides which equipment is the most accurate for a particular product. Any of these methods are much faster than measuring HDT.
Epoxy HDT and Tg measurements are highly correlated. Flexible urethane elastomers exhibit no meaningful HDT or Tg numbers. Rigid urethanes are measured by both methods, with HDT giving more consistent and relevant results.
Can A Tool Be Used Above Its Glass Transition Or Heat Deflection Temperature?
An epoxy tool will not fall apart, or be unusable, because it was heated above the HDT or Tg point. Fiber-reinforced laminates can operate at higher temperatures. Just be aware that the HDT or Tg point is where mechanical properties start to drop at an increasing rate. Most tools are over designed and are much stronger than need be. It is like a yellow caution light warning of trouble ahead.
The Tg point is important for tight-tolerance tools since the coefficient of thermal expansion increases at that temperature. Molding parts at temperatures over the tool’s Tg can cause dimensional problems.
How Important Is Post-Curing For High-Temperature Tooling?
Many of our high-temperature systems are what we call “Room-Temp-Set, Hi-Temp Systems”. This means the resin will cure enough at room temperature for the tool to be removed from the pattern and be given an unsupported post-cure. At this point the material does not have its high-temperature properties.
These types of resin systems have components that cure hard at low temperatures, to give enough strength for demolding. Other components do not crosslink until they see higher temperatures. The first time the resin sees increased temperatures, it softens as crosslinking occurs. It is best to let this reaction happen under controlled temperature increases, without loading the tool. Once the tool has been properly post-cured, it can be exposed to higher temperatures without softening.
Can Epoxies Be Bonded To Cured Epoxy?
Epoxies do not bond well to each other if one surface has cured to a hard glazed-over condition. The glazed surface must be roughed up by sanding, before applying fresh resin. If left unsanded, the bond will be tenuous and likely to separate or chip off at some later time. An example of this is laminating behind a glazed surface coat. If the back of the surface coat is not sanded, it will separate from the laminate when either being exposed to high temperatures or being flexed during service.
How Can Empty Containers Be Disposed?
Click to download the PDF version of
Report From Department of Toxic Substances Control
